Article Text
Abstract
Introduction Several UK military expeditions have successfully used physiological sensors to monitor participant’s physiological responses to challenging environmental conditions. This article describes the development and trial of a multimodal wearable biosensor that was used during the first all-female unassisted ski crossing of the Antarctic land mass. The project successfully transmitted remote real-time physiological data back to the UK. The ergonomic and technical lessons identified have informed recommendations for future wearable devices.
Method The biosensor devices were designed to be continuously worn against the skin and capture: HR, ECG, body surface temperature, bioimpedance, perspiration pH, sodium, lactate and glucose. The data were transmitted from the devices to an android smartphone using near-field technology. A custom-built App running on an android smartphone managed the secure transmission of the data to a UK research centre, using a commercially available satellite transceiver.
Results Real-time physiological data, captured by the multimodal device, was successfully transmitted back to a UK research control centre on 6 occasions. Postexpedition feedback from the participants has contributed to the ergonomic and technical refinement of the next generation of devices.
Conclusion The future success of wearable technologies lies in establishing clinical confidence in the quality of the measured data and the accurate interpretation of those data in the context of the individual, the environment and activity being undertaken. In the near future, wearable physiological monitoring could improve point-of-care diagnostic accuracy and inform critical medical and command decisions.
- physiology
- biotechnology & bioinformatics
- telemedicine
- primary care
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
Using a wearable multimodal sensor, a smartphone and a commercially available satellite transceiver, it has been possible to remotely monitor physiology in real-time.
The identification and sensing of specific diagnostic biomarkers in blood, perspiration, saliva or interstitial fluid at point of care may improve diagnostic accuracy.
Physiological responses to many extreme environments remains poorly understood.
The future success of wearable technologies lies in establishing clinical confidence in the quality of the measured data and the accurate interpretation of those data.
The provision of real-time artificial intelligence-interpreted physiological data could potentially improve diagnostic accuracy and inform critical decisions.
Introduction
The impact of prolonged arduous military training on female physiology and psychology is underinvestigated, and the underlying physiological mechanisms remain poorly understood. The Women in Ground Close Combat review1 highlighted the need for research to precisely describe and explain the physiological effects of arduous training in order to quantify the risks to women of ground close combat integration. The paucity of scientific research has in part been hampered by the practical difficulties of obtaining accurate data outside of laboratory conditions. Consequently, there is little published data on the real-time adaptive changes of human performance parameters such as HR, oxygen saturation, lactate and glucose metabolism over prolonged arduous endurance exercise, particularly for women.
In response to the changing spectrum of military engagement, UK military exercises and operations are becoming increasingly expeditionary.2 3 Potentially fragile lines of communication and delayed evacuation to definitive medical care present significant challenges for those delivering remote medical care.4 A pressing and enduring requirement is the ability to rapidly and reliably monitor the physiological status of personnel on operations, in training or, when injured, throughout the medical evacuation chain. Wearable multimodal physiological monitoring devices may offer a solution to this prehospital dilemma.
The continuous, non-invasive monitoring of human performance, health and well-being has long been an aspiration. The past decade has seen a significant growth in the development of wearable physiological monitors in response to the personal fitness and recreational sports sectors.5 Physiological monitoring relies on biological sensors (biosensors) to convert specific physiological behaviour into measurable electrical signals. These physiological parameters may be physical such as HR and skin temperature, or chemical such as glucose or lactate. The chemical signals derived from the analysis of body fluids, for example, blood, sweat or saliva are proving to be more difficult to measure outside of laboratory conditions. Historically, the majority of the commercially available, off-the-shelf technologies have lacked the accuracy, battery life or durability required for rigorous scientific studies in remote arduous environments.6 However, recent advances in materials science, biomedical, micro, nano and communication technologies have addressed many of the early technology shortfalls.7 There is an increasingly diverse array of more accurate, durable biosensors that have the potential to revolutionise access to real-time physiological monitoring.8 9 These technologies are being successfully exploited in space exploration and elite sports to improve human performance and safety.10 Several UK military expeditions have successfully used physiological sensors to monitor participant’s physiological responses to challenging environmental conditions during adventurous expeditions.11 12 The availability of accurate, timely and intelligible physiological data has obvious utility in the military context, in managing human performance, health and well-being and assisting clinicians and commanders in managing uncertainty and thereby improve critical decision making.
This article describes the development and trial of a multimodal wearable biosensor for which there is limited precedent outside of laboratory conditions. The challenges and pitfalls of trying to deliver remote, continuous, real-time physiological monitoring in remote extreme environments are discussed.
Background
During the Antarctica summer season of November 17 to January 18, the first all-female Army expedition known as exercise Ice Maiden successfully skied unassisted across the Antarctic land mass in a record breaking 62 days.13 The six participants, known as Ice Maidens, volunteered to wear physiological monitoring devices and also participate in concurrent endocrine,14 energy expenditure15 and psychological16 studies during this arduous endurance expedition.
A collaborative research venture between the Academic Department of Military General Practice and the Hamlyn Institute for Global Health Innovation led to the development of a wearable multimodal biosensor that was integrated into the participant’s sports bras. This novel biosensor combined both physical and chemical sensors that were capable of continuously monitoring and storing HR, body temperature, ECG (single lead), sodium, glucose, lactate and pH for a period of up to 25 days. The Hamlyn team had successfully developed individual biochemical sensors but had never combined all the sensors into a single device for use outside of a laboratory setting.17 The device included an accelerometer and exploited near-field communication (NFC) technology to enable wireless data transfer to a handheld electronic device. Samples of the monitored data were encrypted and securely transmitted back to the UK research base using a commercially available satellite transceiver.
Methodology—device specification and trials
The development strategy was influenced by time constraints and the limited availability of in-house technical expertise. The initial plan was to trial commercially available off-the-shelf monitoring devices in order to reduce development time, and to optimise the testing opportunities available during the final preparation phases of the expedition. Analysis of the expedition plan produced a set of technical and user requirements that would maximise the scientific endeavour, while minimising the ergonomic, logistical and administrative impact on the participants. Table 1 summarises some of the major factors that informed the initial selection criteria for the devices and subsequently the design specification for the custom-built wearable device.
Summary of ergonomic and technical considerations
A universal challenge for expeditions of this nature remains access to power required to run communication, navigation and satellite equipment. The generally accepted solution for this environment is to use light weight solar panels and power banks, which all add to the load that must be either carried or pulled. In the context of this expedition, the continuous daylight, weather permitting, allowed recharging on the move as well as during the rest periods.
The ideal monitoring device would allow positioning on the body to assure reliable and accurate physiological monitoring and be small enough to be unnoticed by the wearer. It would have sufficient power to monitor and store the physiological data continuously for the duration of the expedition and allow wireless exchange of data with a satellite transceiver requiring minimal interaction by the user. A number of commercial off-the-shelf sensor devices were reviewed and some trialled during the preparatory training exercises. All the devices had limitations with battery life and required recharging on a daily basis. This requirement was perceived by the participants as having a moderate impact on their evening administration and rest periods. Devices held in place by straps or integrated into specific garments worn across the chest were deemed too cumbersome to integrate with existing protective clothing systems and harnesses.
The final solution was the bespoke design and production of a flexible multimodal biosensor that was robust enough to endure continuous upper body movement but flexible enough to be sewn into the participants’ own sports bras and be worn unnoticed. The device incorporates acquisition channels devoted to the measurement of both physical parameters (ECG, body impedance, temperature and motion) and electrochemical analytes (sweat pH, sodium, lactate and glucose) as illustrated in Figure 1. The ECG signal is recorded on a single bipolar lead through conductive textile electrodes, followed by signal amplification and digitation, with a resolution of 62 μV (range between 0 and 15 mV) and a sampling rate of 50 samples per second. The electrochemical analytes, pH and sodium are measured by a voltammeter transduction mechanism once per second. The concentration of protons (pH) and sodium ions increase the voltage potential between a reference electrode (biassed at 0.7 V) and the respective functionalised working electrode with high sensitivity for pH and sodium. The sensitivity achieved for each analyte, as measured experimentally is 50 mV/unit for pH (pH range 4–9) and 20 mV/mM for sodium at concentrations up to 25 mM. Glucose and lactate are measured amperometrically by selective membranes deposited on the working electrodes for the two analytes. Each generates an ionic current that flows from the reference electrode (same as in the previous voltammeter measurements) to the respective working electrode, reaching sensitivities of 0.8and 2 μA/mM for lactate and glucose, in the concentration range of 25 mM.7
(A) Multimodal biosensor, (B) device positioning, smart phone App and satellite transceiver. GLU, glucose; LAC, lactate; NFC, near-field communication; USB, Universal Serial Bus.
The central microcontroller embedded on device’s electronics is responsible for data digitation, storage inside a flash memory and transfer via the NFC or Universal Serial Bus interfaces to an android smartphone. Due to the asynchronous operation mode selected for the device during the expeditionary trial, a complete data stream is only acquired once every 10 min (recording session), followed by data storage and device shutdown for the remaining 9 min in order to save battery power. This sampling strategy maximised the battery life to approximately 25 days. In order to avoid having to recharge the devices each participant was supplied with three sports bras, each with an integrated monitoring device to cover the duration of each of the three phases of the expedition. A detailed technical description of the device that includes performance parameters, resolution of data collected for the physical parameters, the dynamic ranges of the chemical sensors and references ranges compared with standard measures are described by Rosa et al 17 and Gill et al. 18
Methodology—data collection, storage and transmission
The crossing attempt started at the foot of the Harold Byrd Mountains on the Ross Ice Shelf on 20 November 2017. The Ice Maidens arrived at the Amundsen-Scott South Pole Station on 17 December 2017, having crossed the Leverett Glacier pulling a starting load of 74 kg. The ascent from sea level to 2800 m on the polar plateau took 26 days with temperatures ranging from −41°C to −8°C. The maximum wind speed was 45 mph. The total distance covered was 1713 km which took 62 days to complete. The average distance travelled was 28 km/day during an average 9-hour ski session. The daily resting and administration period was 12±2 hours. It was during these administration periods that the data were uploaded from the wearable devices and transmitted back to the UK research base.
Prior to the start of the crossing, baseline physiological measurements of all the participants were recorded to confirm satisfactory hardware and software configuration and provide a control for subsequent data comparison. Physiological monitoring took place for the duration of the expedition. Each wearable device was used continuously for a period of approximately 3 weeks (longest 27 days, shortest 15 days). The data remained stored on each device and the intention was to harvest it on return to the UK research base. Each participant’s smartphone had a purpose-built communication App installed. A data sample could be transferred to the smartphone by activating the App and holding the phone in close proximity to the monitoring device. The App automatically configured an encrypted data file to include a unique participant identifier, the sampled physiological data and geo-positional information from the phone. The unique participant identifier was allocated randomly to each participant prior to the expedition. In accordance with ethical guidance, the data remained anonymised until the expedition was completed. It was considered inappropriate to unduly influence medical or expedition decisions based on an unproven technology and the paucity of scientific evidence available to interpret the physiological data. A wireless interface between the smartphone and an Iridium-go satellite transceiver allowed an encrypted data file transfer to the UK research base. The plan was, weather permitting, to transmit the information on a weekly basis. Data files received at the UK research base were checked for integrity then securely stored for postexpedition analysis.
Participation in this study was voluntary and independent of the expedition.
Results
Pre-expedition testing of the device was performed under laboratory conditions in continuous acquisition mode (without device shutdown), in order to assess response to physical exercise. The activity included a 10 min period of indoors cycling followed by an equivalent rest period. Results for one volunteer are shown in Figure 2A–E. The physical and electrochemical channels were characterised and compared with other certified measurement devices. EGC was compared with a commercial scope obtaining a signal error of 7% (or 1.1 mV); impedance employed a commercial impedance analyser with amplitude error of 3% (30 kΩ); temperature with a thermometer calibrator with an error of 5% (1.5oC); the electrochemical channels delivered an average signal error of 4% (2 mV), 7% (1.4 mV), 6% (4.8 pA) and 10% (0.2 μA) for pH, sodium, lactate and glucose analytes, respectively.7 17
(A–E) Laboratory performance test results. (F) Data parameter trends reconstructed from satellite transmissions for one volunteer.
Device function and battery life
Fourteen of the 18 devices functioned for the duration of the expedition. The power supply to four of the devices were damaged from overstretching of the wires. This was the result of pre-expedition weight gain of the participants, a factor that was not anticipated during the original design, and not experienced during the trials. All the batteries functioned without fault for the duration of the expedition and there was no reported difficulty charging the mobile devices or the satellite transceivers using the power banks.
Postexpedition analysis of the devices revealed that 30% of them suffered considerable structural damage which prevented recovery of the stored data, while in the remaining 70% only some corrupted data streams were effectively recovered from the flash memory. Factors that affected data reliability and integrity of data storage, which were not identified during testing included the transportation of the worn devices in subzero temperature conditions, and intense body movements affecting the digital signals responsible for the communication protocol between the microcontroller and memory, especially over the flexible substrate. It has not been possible therefore to accurately interpret the participant’s physiological adaption to prolonged physical exertion.
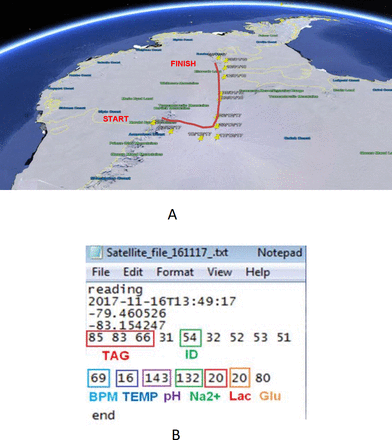
Data were successfully transmitted to the UK research base via satellite on seven occasions. Figure 2F illustrates the data trends reconstructed from the satellite transmissions for one volunteer relative to their baseline recordings. Severe weather conditions interfered with the satellite transmissions during the first phase of the expedition (21 days). Body motion is absent from the graph as the accelerometer data were not sent through the satellite transmissions because of the high number of data points involved. The progress of the expedition was tracked in real time by using GSM data from the smartphone (Figure 3). These data were used to index environmental conditions, such as altitude, wind speed and direction for each particular location. This information would provide valuable context in which to interpret the participant’s physiological responses.
Antarctic route map with overlaid geo-positioning and a satellite file with a data sample.
Ergonomic factors
All participants reported acceptable levels of comfort, most were unaware of the device during the exercise phases. The participants reported occasional discomfort if they rolled onto their left side during the rest periods. Movement friction eroded some of the protective coating off the perspiration sensors which led to minor irritability and skin excoriation for two of the participants but neither reported any significant sequalae. Postexpedition feedback from the participants regarding device improvements included a light to confirm that device is functioning correctly, visual feedback of physical performance and more device control features built into the App. Considerations for future human-device interfaces will include either direct attachment to the skin or implantation. These approaches would reduce skin friction and potentially unwanted motion artefact.
Discussion
This was a pilot study of a novel multimodal physiological monitoring device. The primary objective of study was to capture physiological data sampled from the participants and securely transmit the data back to the research base in the UK. This was successfully undertaken on seven occasions during the expedition. Regrettably, the secondary aim of analysing the data set of the participant’s physiological performance over the duration of the expedition was not achieved.
The analysis of the sweat analytes sodium, lactate and glucose outside of the laboratory remains challenging. Only impedance measuring supports real-time in situ extensive periods of continuous measurement in remote locations. The quality of impedance measurement is constrained by the relatively simplified electronics and flexible circuitry required within the device and this in turn leaves the sampling channels prone to saturation. Further studies are required to understand the full impact of these constraints on the device’s accuracy and duration.
With hindsight, recording a 30 second data sample every 10 min for the duration of the expedition was an overambitious aspiration, which had implications for battery life, the design and performance of the microprocessors and storage devices. This sample rate produced in the order of 105 data points per parameter measured. From a monitoring and diagnostic perspective, the high data sample rates were probably unnecessary for this type of endurance activity and risked obscuring important trends by the sheer volume of data. In future, the choice of data sampling rates will consider the mode of surveillance, the physiological parameter, the type and intensity of activity being undertaken and the potential impact of the environmental conditions. An additional level of sophistication to future devices would be the inclusion of real-time data analysis that could be used to control sample rates. Significant deviations from expected physiology, changes in activity level or elevated environment stressors could be used to trigger an increased sampling rate in one or more of the parameters.
The challenge of what constitutes a ‘significant’ or ‘normal’ physiological response in the context of the population at risk, the activity being undertaken and the environment is complex and largely remains unanswered. The investigation by Boos et al exemplifies this diagnostic dilemma. Postexpedition analysis of cardiac recordings identified significant cardiac arrhythmias and, in some cases, pauses of up to 7 s duration in a fit and well population at very high altitudes.12 A clinician observing these data remotely would likely recommend abandoning such attempts based on their experiences of ‘normal’ ECG recordings at sea-level. Stacy et al elaborate on the issues of ‘real-time’ monitoring in healthy military personnel by introducing additional dimensions concerning data accuracy, interpretation, ownership and patient autonomy. The authors point out that the risk of misinterpretation of the physiological data that lies outside of what is deemed ‘normal’ physiology could compromise military or expeditionary activity inappropriately, infer inappropriate employment constraints or worse, lead to harmful false reassurance.19
Therefore, the inferred research strategy must be to develop a more sophisticated understanding of human physiological responses to physical and disease stressors. Identifying the appropriate parameters that should be measured and validating the physiological models are implicit to this task. Wearable technologies may contribute to the prevention, detection and treatment of illness or injury. They may also be used to optimise physical performance. However, with such a broad spectrum of applications, it is unlikely that there will be a unique group of physiological parameters that will provide all the answers, or a single device that could support such a range of functions. Another and perhaps more appealing solution lies with the identification and sensing of specific diagnostic biomarkers in blood, perspiration, saliva or interstitial fluid. Aside from the enormous complexity of isolating unique biomarkers as markers for specific pathologies, the marker must also exist within an accessible body fluid in a timely and measurable concentration There is a potential to gather enormous amounts of real-time data with the associated risk of overwhelming t a medic in circumstances where their bandwidth may already be constrained by external stressors. By introducing data processing and interpretation within the wearable device and also remotely, there is an opportunity to exploit artificial intelligence (AI) machine-based learning to identify meaningful patterns in this big data and assist the medic in the diagnostic and management processes.
User feedback and lessons identified from the pilot study have refined our next generation of wearable devices. Integration of the device into clothing did not appear to cause significant ergonomic issue, however the degree of motion artefact could not be established. Future devices have been adapted to be worn directly on the chest wall, on the wrist or subcutaneously; powered by either battery or near-field technology. The architecture incorporates environmental sensing, a standard array of physiological monitoring, that include HR, surface temperature, ECG and lactate and an interchangeable component that could be configured to measure specific molecular biomarkers. An onboard central processing unit will provide a basic level of data interpretation that supports switching between different monitoring modes as well as more sophisticated data interpretation features. An enhanced smartphone App will allow the user to configure particular arrays of parameters and run device diagnostics. The increased processing capability of the smartphone will be used to deliver more sophisticated data analysis that will be displayed on a real-time dashboard.
Conclusion
Using a wearable multimodal biosensor, a smartphone and a commercially available satellite transceiver, it has been possible to remotely monitor human physiology in real-time.
The future success of wearable technologies lies in establishing clinical confidence in the quality of the measured data and the accurate interpretation of those data in the context of the individual, the environment and activity being undertaken.
The provision of real-time AI-interpreted physiological data would potentially improve diagnostic accuracy and better inform critical medical and command decisions.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
Ethics statements
Patient consent for publication
Ethics approval
Ethical approval was granted by the Ministry of Defence Ethics Committee (842MoDREC/17).
Footnotes
Twitter @jodieblackadder, @icemaidennat
Correction notice This article has been corrected since it appeared Online First. Authors Salzitsa Anastasova and Bruno Gil-Rosa hvae been added, and affiliations updated or consolidated.
Collaborators Professor Guang-Zhong Yang; Dr Salzitsa Anastasova; Dr Bruno Gil-Rosa.
Contributors MS designed, organised the trial and drafted the manuscript. RW was lead investigator and edited the manuscript. JB-C conducted the postexpedition interviews. NT (Ice Maiden) was a participant and advised on the ergonomics and design of the prototype device. She also contributed to the finished manuscript. G-ZY (Medical Director of the Hamlyn Centre, Institute of Global Health Innovation) provided the research team and the resources required to design and build the multimodal device. SA designed and developed the chemical sensors and BG-R designed and developed the integrated circuit, the App and device functionality. SA and BG-R provided the results and technical advice for the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Map disclaimer The depiction of boundaries on this map does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area of its authorities. This map is provided without any warranty of any kind, either express or implied.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.