Abstract
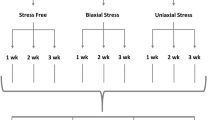
We have measured the dynamics of extracellular matrix consolidation and strengthening by human dermal fibroblasts in hydrated collagen gels. Constraining matrix consolidation between two porous polyethylene posts held rigidly apart set up the mechanical stress which led to the formation of uniaxially oriented fibroblast-populated collagen matrices with a histology resembling a ligament. We measured the mechanical stiffness and tensile strength of these ligament equivalents (LEs) as a function of age at biweekly intervals up to 12 weeks in culture using a mechanical spectrometer customized for performing experiments under physiologic conditions. The LE load-strain curve changed as a function of LE age, increasing in stiffness and exhibiting less plastic-like behavior. At 12 weeks, LEs had acquired up to 30 times the breaking strength of 1-week-old LEs. Matrix strengthening occurred primarily through the formation of BAPN-sensitive, lysyl oxidase catalyzed crosslinks. Sulfated glycosaminoglycan (GAG) content increased monotonically with LE age, reaching levels that are characteristic of ligaments. Cells in the LEs actively incorporated [3H]proline and [35S]sulfate into the extracellular matrix. Over the first three weeks, DNA content increased rapidly but thereafter remained constant. This data represent the first documentation of strengthening kinetics for cell-assembled biopolymer gels and the results suggest that this LE tissue may be a valuable model for studying the cellular processes responsible for tissue growth, repair, and remodeling.
Similar content being viewed by others
References
Aggarwal, A. A syngeneic ligament equivalent, phase I: Mechanical properties of a fibroblast populated collagen lattices. S.B. Thesis, Department of Electrical Engineering and Computer Science, M.I.T., Cambridge, MA; 1987.
Barak, L.S.; Yocum, R.R.; Nothwagel, E.A.; Webb, W.W. Fluorescence staining of the actin cytoskeleton in living cells with 7-nitrobenz-2-oxa-1, 3-diazaole phallacidin. Proc. Natl. Acad. Sci. USA. 77:980–984; 1980.
Bell, E.; Ivarsson, B.; Merrill, C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potentialin vitro. Proc. Natl. Acad. Sci. USA. 76:1274–1278; 1979.
Bell, E.; Ehrlich, H.P.; Sher, S.; Merrill, C.; Sarber, R.; Bull, B.; Nakatsuji, T.; Church, D.; Bittle, D.J. Development and use of a living tissue equivalent. Plast. Reconstr. Surgery 67:386–391; 1981.
Bellows, C.G.; Melcher, A.H.; Aubin, J.E. Association between tension and orientation of periodontal ligament fibroblasts and exogenous collagen fibres in collagen gelsin vitro. J. Cell Sci. 58:125–138; 1982.
Bellows, C.G.; Melcher, A.H.; Aubin, J.E. Contraction and organization of collagen gels by cells cultured from periodontal ligament, gingiva and bone suggest functional differences between cell types. J. Cell Sci. 50:299–314; 1981.
Buttle, D.; Ehrlich, P. Comparative studies of collagen lattice contraction utilizing a normal and a transformed cell line. J. Cell Phys. 116:159–166; 1983.
Chandrakasan, G.; Torchia, D.A.; Piez, K.A. Preparation of intact monomeric collagen from rat tail tendon and skin and the structure of the non-helical ends in solution. J. Biol. Chem. 251:6062–6067; 1976.
Chandrasekhar, S.; Kleinman, H.K.; Hassell, J.R.; Martin, G.R.; Termine, J.D.; Trelstad, R.L. Regulation of Type I collagen fibril assembly by link protein and proteoglycans. Collagen Rel. Res. 4:323–338; 1984.
Coulson, P.C.; Bishop, A.O.; Lenarduzzi, R. Quantitation of cellular deoxyribonucleic acid by flow microfluorometry. J. Histochem. Cytochem. 10:1147–1153; 1977.
Dunn, G.A.; Ebendal, T. Contact guidance on oriented collagen gels. Exp. Cell Res. 111:475–479; 1978.
Ehrlich, H.P.; Borland, K.M.; Muffly, K.E.; Hall, P.F. Contraction of collagen lattice by peritubular cells from rat testes. J. Cell Sci. 82:281–294; 1986.
Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosylaminoglycans by use of dimethylmethylene blue. Bioch. Bioph. Acta 883:173–177; 1986.
Franklin, T.J.; Gregory, H.; Morris, W.P. Acceleration of wound healing by recombinant human urogastrone (EGF). J. Lab. Clin. Med. 108:103–108; 1986.
Goldman, R.D.; Schloss, J.A.; Starger, J.M. Organizational changes of actin like microfilaments during animal cell movement. In: Cell motility. Goldman, R.; Pollard, T.; Rosenbaum, J. (eds.) New York: Cold Spring Harbor Laboratory; 1976: pp. 217–245.
Goss, R.J. Bone and cartilage. In: The physiology of growth. New York: Academic Press; 1978: p. 64.
Grinnell, F.; Lamke, C.R. Reorganization of hydrated collagen lattices by human skin fibroblasts. J. Cell Sci. 66:51–63; 1984.
Guidry, C.; Grinnell, F. Contraction of hydrated collagen gels by fibroblasts: Evidence for two mechanisms by which collagen fibrils are stabilized. Collagen Rel. Res. 6:515–529; 1986.
Guidry, C.; Grinnell, F. Heparin modulates the organization of hydrated collagen gels and inhibits gel contraction by fibroblasts. J. Cell Bio. 104:1097–1103; 1987.
Guidry, C.; Grinnell, F. Studies on the mechanism of hydrated collagen gel reorganization by human skin fibroblasts. J. Cell Sci. 79:67–81; 1985.
Harkness, R.D. Mechanical properties of skin in relationship to its biological function and its chemical components. In: Elden, H.R. (ed.) Biophysical properties of skin. New York: John Wiley & Sons; 1971: pp. 393–436.
Huang, C. Physicochemical studies of collagen and collagen-mucopolysaccharide composite materials. Ph.D. Thesis, Department of Mechanical Engineering, M.I.T., Cambridge, MA; 1974.
Ignotz, R.A.; Massague, J. Transforming growth factor stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. pp. 4337–4345; 1986.
Kagan, H. M. Characterization and regulation of lysyl oxidase. In: Mecham, A.P. (ed.) Regulation of matrix accumulation. New York: Academic Press; 1986.
Kim, Y.J.; Sah, R.L.Y.; Doong, J.-Y.H.; Grodzinsky, A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal. Biochem. 174:168–176; 1988.
Labarca, C.; Paigen, K. A simple, rapid, and sensitive DNA assay procedure. Anal. Biochem. 102:344–352; 1980.
Lackie, J.M. Cell movement and cell behavior. Boston, MA: Allen & Unwin; 1986.
Lee, R.C.; Frank, E.H.; Grodzinsky, A.J.; Roylance, D.K. Oscillatory compressional behavior of articular cartilage and its associated electromechanical properties. J. Biomech. Eng. 103:280–292; 1981.
McLeod, K.J.; Lee, R.C.; Ehrlich, H.P. Frequency dependence of electrical field modulation of fibroblast protein synthesis. Science. 236:1465–1469; 1987.
Narayanan, A.S.; Siegel, R.C.; Martin, G.R., On the inhibition of lysyl oxidase by b-aminopropionitrile. Bioch. & Bioph. Res. Comm. 46:746–751; 1972.
Nimni, M.E.; Harkness, R.D. Molecular structure and functions of collagen. In: Nimni, M.E. (ed.) Collagen. Vol. I. Boca Raton, FL: CRC Press, Inc.; 1988: pp. 1–77.
Nusgens, B.; Merrill, C.; Lapierre, C.; Bell, E. Collagen biosynthesis by cells in a tissue equivalent matrixin vitro. Collagen Rel. Res. 4:351–364; 1984.
Reitzer, L.J.; Wice, B.M.; Kennell, D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J. Biol. Chem. 254:2669–2676; 1979.
Roberts, A.B.; Sporn, M.B.; Assoian, R.K.; Smith, J.M.; Roche, N.S.; Wakefield, L.M.; Heine, U.I.; Liotta, L.A.; Falanga, R.; Kehrl, J.H.; Fauci, A.S. Transforming growth factor type: Rapid induction of fibrosis and angiogenesisin vivo and stimulation of collagen formationin vitro. Proceedings of the National Academy of Sciences. 83:4167; 1986.
Scott, J.E. Proteoglycan-fibrillar collagen interactions. Biochem J. 252:313–323; 1988.
Silver, F.H. Biological materials: Structure, mechanical properties, and modeling of soft tissues. New York: New York University Press; 1987.
Skalak, R.; Chien, S. Hand book of bioengineering. Chapters 4, 6, 11, and 16. New York: McGraw Hill Book Company; 1987.
Snowden, J.M.; Swann, D.A. Effects of glycosaminoglycans and proteoglycan on thein vitro assembly and thermal stability of collagen fibrils. Biopolymers. 19:767–780; 1980.
Stopak, D.; Harris, A.K. Connective tissue morphogenesis by fibroblast traction. Dev. Bio. 90:383–398; 1981.
Thomas, J. Nutrients, oxygen and pH in mammalian cell technology. Boston, MA: Butterworths; 1986.
Tomasek, J.J.; Hay, E.D. Analysis of the role of microfilaments and microtubules in acquisition of bipolarity and elongation of fibroblasts in hydrated collagen gels. J. Cell Biol. 99:536–549; 1984.
Tomasek, J.J. A serum factor promotes the generation of tension by fibroblasts in attached collagen lattices. J. Cell Biol. 111:148a: 1990.
Trelstad, R.L.; Silver, F.H. Matrix assembly. In: Hay, E. (ed.) Cell biology of extracellular matrix. New York: Plenum Press; 1981: pp. 39–63.
Weiland, T.; Faulstich, H. Amatoxins, phallatoxine, phallolysin and atamanide: The biologically active components of poisonousAmanita mushrooms. CRC Crit. Rev. Biochem. 5:185–260; 1978.
Weinberg, C.B.; Bell, E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 231:397–400; 1986.
Weinberg, C.B.; Bell, E. Regulation of proliferation of bovine aortic endothelial cells, smooth muscle cells, and adventitial fibroblasts in collagen lattices. J. Cell. Physiol. 122:410–414; 1985.
Weiss, P. Erzwingung elementarer strukturver schiedenheiten amin vitro wachsenden gewebe. Wilhelm Roux's Arch. 116: 438–554; 1929.
Weiss, P.In vitro experiments on the factors determining the course of the outgrowing nerve fiber. J. Exp. Zool. 68: 393–448; 1934a.
Weiss, P. Secretory activity of the inner layer of the embryonic mid-brain of the chick, as revealed by tissue culture. Anat. Rec. 58:299–302; 1934b.
Wolff, J. The law of bone remodelling. Translated by P. Maquet and R. Furlong. Berlin: Springer-Verlag; 1986.
Woo, S.L.Y.; Buckwalter, J.A. Inquiry and repair of the musculoskeletal soft tissues. Park Ridge, IL: American Academy of Orthopedic Surgeons; 1988.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Huang, D., Chang, T.R., Aggarwal, A. et al. Mechanisms and dynamics of mechanical strengthening in ligament-equivalent fibroblast-populated collagen matrices. Ann Biomed Eng 21, 289–305 (1993). https://doi.org/10.1007/BF02368184
Issue Date:
DOI: https://doi.org/10.1007/BF02368184